 Physicists can't use light to
explore atomic and sub-atomic structures because
light's wavelength is too long. However, since
ALL particles have wave properties,
physicists can use particles as their probes.
In order to see the smallest
particles, physicists need a
particle with the shortest possible wavelength possible.
However, most of the particles around us in the
natural world have fairly long wavelengths.
How do physicists decrease a particle's
wavelength so that it can be used as a probe?
Physicists can't use light to
explore atomic and sub-atomic structures because
light's wavelength is too long. However, since
ALL particles have wave properties,
physicists can use particles as their probes.
In order to see the smallest
particles, physicists need a
particle with the shortest possible wavelength possible.
However, most of the particles around us in the
natural world have fairly long wavelengths.
How do physicists decrease a particle's
wavelength so that it can be used as a probe?
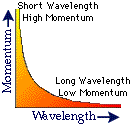
![]() A
particle's momentum and its wavelength are inversely related.
A
particle's momentum and its wavelength are inversely related.
High-energy physicists apply this principle when
they use particle accelerators
to increase the momentum of a probing particle,
thus decreasing its wavelength.
Steps: