 Around 1900, people thought that atoms were like soft balls,
with small positive and negative particles embedded in them.
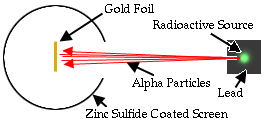
In 1909, Rutherford, supervising Geiger and Marsden, tested the
validity of this theory in his now-famous gold-foil experiment.
Around 1900, people thought that atoms were like soft balls,
with small positive and negative particles embedded in them.
In 1909, Rutherford, supervising Geiger and Marsden, tested the
validity of this theory in his now-famous gold-foil experiment.
The experiment was quite simple: a radioactive source
shot a stream
of alpha particles.
They were shot at very thin gold foil.
Alpha particles would make little flashes of light
where they hit the screen.
They expected that the alpha
particles would have no problem zipping straight
through gold foil that was only a few atoms thick.
|
|
|







